DT120 ODT
Science of DT120
DT120 orally disintegrating tablet (ODT) is our pharmaceutically optimized proprietary formulation of lysergide (LSD) D-tartrate. DT120 is an ergoline derivative belonging to the group of classic serotonergic psychedelics, which acts as a partial agonist at specific serotonin receptors, causing its distinctive transient perceptual and emotional effects.
Mechanism of Action
DT120 is thought to increase connections between areas of the brain that aren’t normally connected, enabling changes that could treat depression, anxiety and other brain health disorders
Lead candidate with potential benefits across multiple therapeutic areas
Designed for Optimized Delivery
DT120 ODT
DT120 orally disintegrating tablet (ODT) is our pharmaceutically optimized proprietary formulation of lysergide (LSD) D-tartrate. DT120 ODT is an advanced formulation incorporating Catalent’s Zydis® fast-dissolve technology. This formulation is designed to deliver several unique advantages:
- Faster absorption and faster onset of transient cognitive, perceptual and affective changes
- Improved bioavailability
- Lower incidence of gastrointestinal side effects
In clinical trials, DT120 has shown positive effects in reducing symptoms of anxiety and depression after just a single administration. These effects can be rapid and durable, beginning as quickly as the day after treatment and maintaining for at least 3 months.1

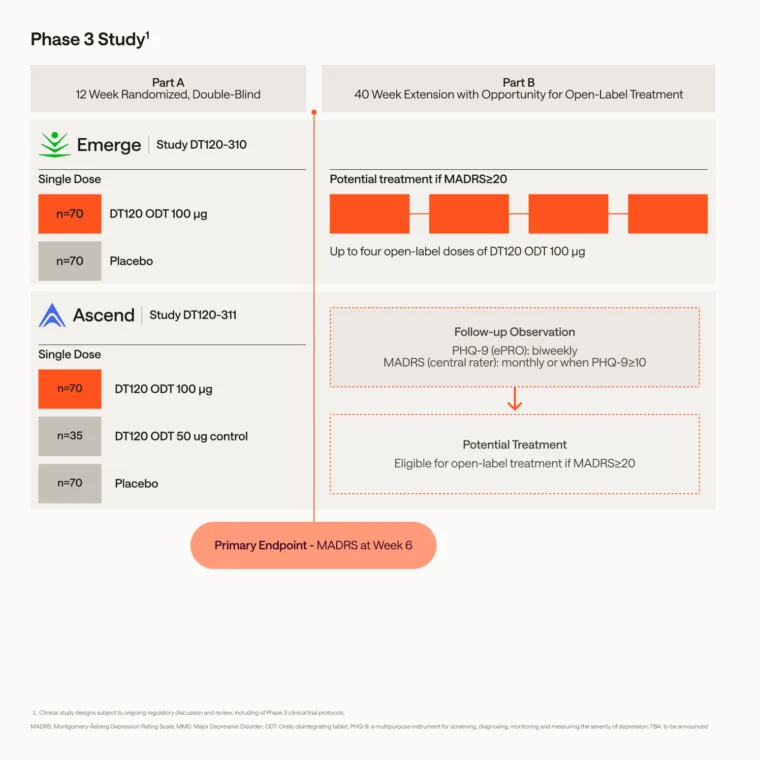
Phase 3 Study Designs
DT120 ODT for GAD
Two Phase 3 registrational trials are evaluating the efficacy and safety of DT120 ODT in the treatment of adults living with GAD versus placebo. Voyage, the first study, is expected to enroll approximately 200 participants in the U.S. Panorama, the second Phase 3 study, will be conducted in the U.S. and Europe, and is expected to enroll 250 participants.
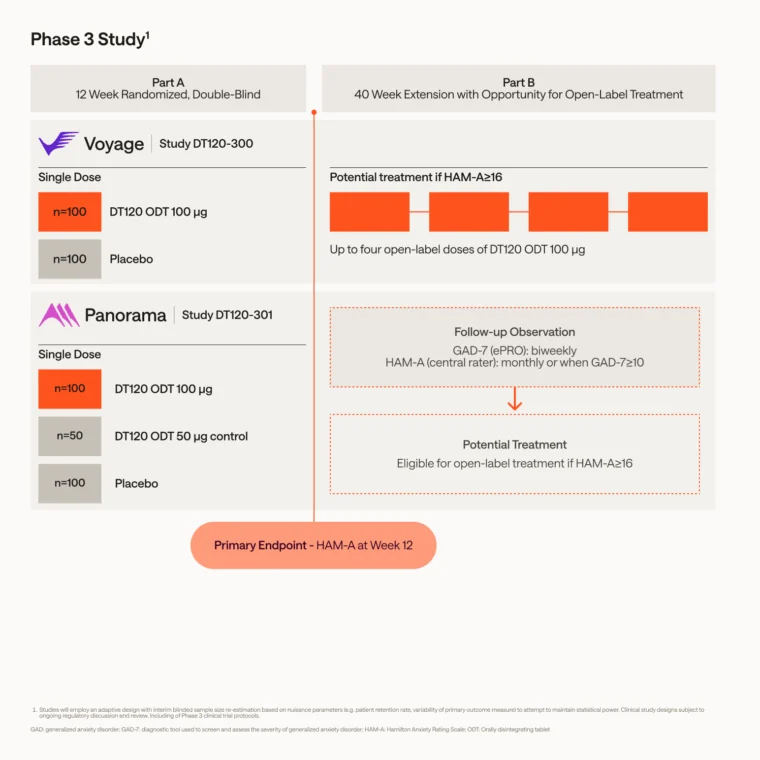
Phase 3 Study Designs
DT120 ODT for MDD
Emerge, the first Phase 3 trial in MDD is evaluating the efficacy and safety of DT120 ODT in the treatment of adults living with MDD versus placebo. Emerge is expected to enroll approximately 140 participants in the U.S.
Ascend, our second phase 3 pivotal study of DT120 ODT in MDD, is expected to initiate in mid-2026. Ascend is expected to enroll at least 175 participants.
DT120 granted Breakthrough Therapy Designation from FDA
Based on the significant unmet medical need in the treatment of GAD and the clinical data from our Phase 2b study, the U.S. Food & Drug Administration has granted Breakthrough Therapy Designation for the DT120 program in GAD.

Be a part of what’s next
We are working to turn breakthroughs in the lab into breakthroughs in the lives of people who are most in need. By participating in a clinical trial, you may play a crucial role in improving care and developing potential new treatments that may one day change lives, including your own.

Precise science. Boundless impact.
- Paula L. Jacobsen, Ph.D.; Jamie M. Freedman, BS; Jamileh Jamison, MD, MS; Sarah M. Karas, PsyD; Daniel R. Karlin, MD, MA; Reid Robison, MD (2024). Rapid and Durable Response to a Single Dose of MM120 (Lysergide) in Generalized Anxiety Disorder: A Dose Optimization Study.
The therapies we are investigating are not approved by the FDA or any other countries’ Health Authorities. This means their safety and efficacy are still being evaluated and have not been established.